Charge
As mentioned in the last section, there are two kinds of charge: positive and negative. (You can blame Benjamin Franklin for deciding which was which. It turns out your life as a physicist would be easier if he had chosen the other way. We call electrons negative, but they are the charges that move around and create currents. It would be a little nicer if they were the positive charges. But we are stuck with it.)
Direction of force
You've probably heard the old adage "opposites attract." Well, this is true of electric charge. Opposite charges attract each other, that is, exert forces on each other that point from one charge to the other charge. If opposite charges started from rest and were released, they would travel straight toward each other with increasing speed (like an object falling from rest toward the ground).
In the figures below, it doesn't matter which is positive, red or blue. The only thing that matters is that the two charges attract each other. (The arrows, which will represent force, point along the line joining the two charges, and toward the other charge.) I have to admit that I think of red as negative, and when it matters, that's the choice I'll make. But for now, it doesn't make the slightest bit of difference.
![]()
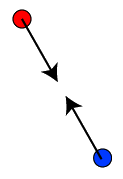
![]()
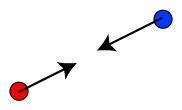
Like charges, either both positive or both negative, repel each other as shown below.
- Opposite charges attract
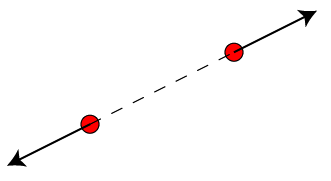
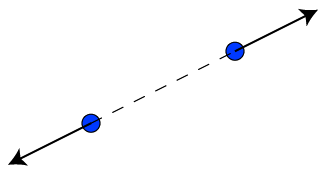
- Like charges repel
Charge Conservation
Charge is conserved. (Recall that a conservation law refers to a quantity that remains the same (for a closed system) over time.) The total amount of charge in the universe remains the same. You can move charges around; even by simply rubbing two objects together you can rub some electrons off one object and onto the other, but they are not destroyed--they simply move. If you do something to create, or destroy, +1 unit of charge, you will necessarily create, or destroy, -1 unit of charge at the same time.
Charge Quantization
Charge is quantized. That means it comes in integer multiples of the base unit. The base unit for charge is $e$, the magnitude of the charge on the electron. (Already you wish the charge of the electron were positive so I didn't have to say magnitude.) So an electron has a charge of -$e$, and a proton has a charge of +$e$. $$e = 1.6 \times 10^{-19}\mbox{C}$$ Charge quantization means that you will never see a charge of $\frac{1}{2}e$ or $0.1e$ or any other fraction. (Of course you can see charges of .000001C, because a Coulomb is $10^{19}$ times larger than the charge on an electron, so .000001C is still many many times the charge of an electron.)
Quarks
You may already know something about quarks, and if you don't, it's still interesting enough to mention here. Physicists have a model of fundamental particles and how they are made. Quarks are an important part of that model. A proton is made up of three quarks: two up's and a down. (Those are actually the names of two different kinds of quarks: up and down.) An up quark has a charge of $\frac{1}{3}e$ and a down quark has a charge of -$\frac{2}{3}e$.
At this point you should be thinking, "Wait a second, you just told me that charge was quantized in units of $e$!" Good point--I did. It turns out no one has ever managed to isolate a single quark.
A proton is made up of three quarks (uud) and a neutron is also made up of three quarks (udd). There are other particles made of two quarks (actually a quark and an anti-quark). However, if you try to pull one quark out of a proton or neutron or any other particle, you have to put in so much energy to do it, that you actually create a new pair of quarks! So any particle that you ever see is always made up of more than one quark, and the charge is always a multiple of $e$. Pretty cool, huh.